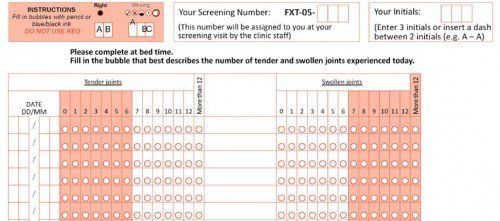
Drug Trials
Another interesting application for OMR technology that MurrayData have been involved in is drug trials. Sometimes also referred to as ‘clinical study patient diary card’ forms, these are given to patients at the beginning of a drug trial in order to record symptoms and changes during the number of weeks they will participate in the study.
This type of application is normally provided as a ‘bureau’ or scanning service, as it is usually more economical to have MurrayData do all the scanning of forms and then sending a CSV or Excel file as captured data to the clinical study organisation.